Fungal Farming in Leafcutter Ants
Bio342 Fall 2010
by Emily Crotteau
Phylogeny:
Phylogeny attempts to provide a description of the evolutionary history of a species based evidence from fossil precursors and DNA evidence. In looking at the relationship between attine ants and fungus, the question of phylogeny must look beyond the evolution of a single species and encompass the evolution of their association. In short, we are asking, what is the genealogy of this symbiosis?
Phylogeny of Fungal Farming
In 50 million years of shared evolutionary history, the basal attine lineages, called the lower-attines, have shifted between two main cultivar clades. Species of this group typically collects frass, dead insects, and fallen plant material for their gardens, and are relatively monomorphic (14). Basal attine cultivars are frequently free-living, showing little adaptive specialization to their hosts, whereas leaf-cutter and other higher- attine cultivars are generally in reciprocally obligate relationships with their hosts, uniquely adapted to their situation and consequently dependent on the relationship for survival. No known fungus growing ants have reverted to their ancestral state of "hunter-gatherer" food harvesting, indicating that this behavior is an example of "irreversible evolution of novel trait syndromes" (5).
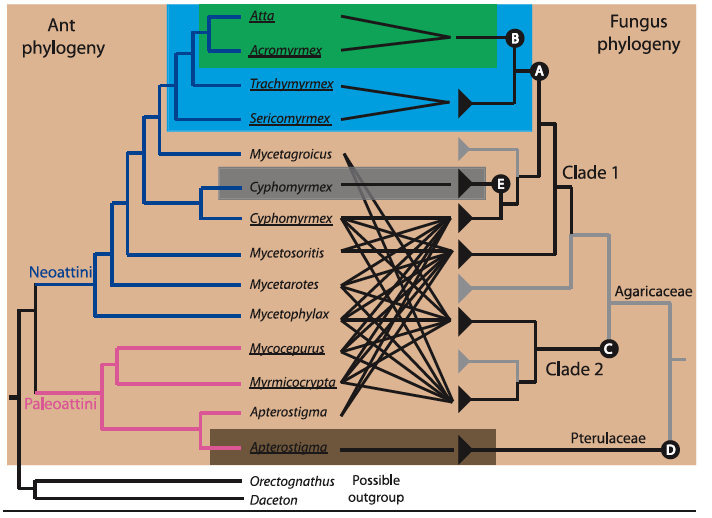
Figure 2. Genus-level diagram of the attine ants combined with fungal cultivar phylogeny, with black lines indicating mutualistic relationships.
Background color indicates the five major groups of attine ants.
Green: leaf-cutter ants, a subset of the higher-attines, which use freshly cut leaves as fungal substrate
Blue: other higher-attines, which use freshly shed flowers, insect frass, and soft leaf fragments for substrate
Light brown: lower-attines, which use insect frass, dry plant material, seed, and seed husks as substrate
Gray: yeast-farming attines
Dark brown: coral-fungus farming ants, which, like the lower-attines, use insect frass, dry plant material, seed, and seed husks as substrate
The fungal phylogeny is divided into two major agaricaceous (as opposed to free-living) clades, the original Clade 1 and secondary Clade 2, with the Pterulaceae family consisting of an additional secondary event unique to the coral-fungi clade. Fungal symbiont branchs are indicated in black, whereas free-living branches are designated by gray. [Image courtesy of De Fine Licht, H. H. (2010)]
The letters indicated in the diagram above indicate specific evolutionary events in the development of the symbiotic relationship between ants and fungus.
A. Fungal cultivars develop swollen hyphal tips, gongylidia, which provide nutrition in the ancestors of the cultivars of higher-attine ants, including leaf cutters.
B. Unification towards a single species of symbiont, Leucocoprinus gongylophorus, specially adapted for interrelationship with leaf cutter ants, a shift away from the diverse range of free-living fungi cultivated in ancestral species.
C. Development of distantly-related agaricaceous fungi which form symbiotic relationships to certain lower-attine lineages. This is the second time a branch of fungus has ceased to be free-living in favor of a mutualistic relationship with ants.
D. Completely unrelated development of pterulaceous (coral) fungi in relationship the genus Apterostigma. This is the third derived instance of the evoltuion of ant-fungus mutualism.
E. Transition to farming of single cell basidiomycete yeast rather than mycelial growth by the genus Cyphomyrmex.
In summary, the independent evolution of mutualistic relationships between ants and members of the fungi kingdom is believed to have occurred at least four times throughout the shared history of the neoattini and paleoattini tribes. One distinguishing event in the development of the higher-attines was the emergence of exclusive cultivation of a single symbiont, as opposed to the myriad relationships between ants and fungi that flourish among the lower-attines. While significant research has been devoted to elucidating the key events of ant-fungal co-evolution, few models have been proposed to explain the basis of the transitions, though it appears that the driving factor has been unlocking a greater diversity nutrient sources for the benefit of both species.
The history of fungal symbiont evolution can be traced in specific modifications to the enzyme profile of the fungi, reflective of changes in cultivar genotype and changes in the substrate used as manure in the gardens, which is ultimately a result of ant foraging strategies. Lower-attine agriculture shows significantly less endo-protease activity, with enzyme action primarily targeted toward the partial degration of plant walls, reflective of the plesiomorphic state of free-living, nondomesticated fungi (12). Higher-attine cultivars show increased activity of endo-protease and other protein- and starch-digesting enzymes, which is believed to target the degradation of fresh leaves, flowers, and fruits, rather than dead material as is common among the lower-attines. In fact, studies have shown that "the leaf-cutting ant cultivar has a unique enzymatic profile, which appears to be ideally suited to degrading freshly cut plant material" (5). In a sense, then, the evolution of ant-fungus mutualism can be viewed as a co-development of ant foraging techniques and fungal digestive capabilities ultimately enabling both species to derive vast nutritional benefit from the otherwise inaccessable, undigestable cellulose and starch contained in leaves.