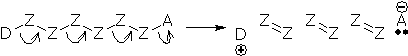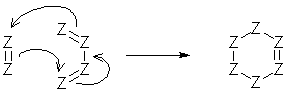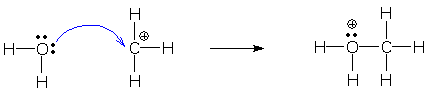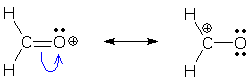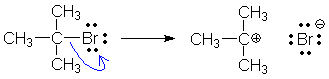
|
|

|
Curved Arrows: Formal Charge Calculation Electron movements may change formal charges. You can quickly write the new formal charges, however, if you pay attention to arrow locations and follow these simple rules:
Most electron movements are represented by chains of arrows. A chain consists of a tail atom (D), a head atom (A), and some tail+head atoms (Z) in between. There is always a net transfer of one electron from the tail to the head (from D to A), but the other atoms do not gain or lose electrons. (Note: organic chemists commonly use D and A to represent generalized electron donor and electron acceptor atoms.)
Some electron movements are represented by rings of arrows. A true ring contains only tail+head atoms, and no changes in formal charge occur.
Examples The following reaction shows the shortest chain possible (one arrow). One electron shifts from O (tail) to C (head atom), so we add +1 to the formal charge on O and -1 to the charge on C.
The next example shows two resonance forms, again related by the shortest chain of arrows possible: one arrow. Again, we add +1 to the tail atom's (C) charge and -1 to the head atom's (O) charge.
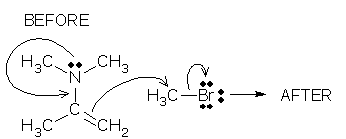
Is it completely obvious that O is the tail atom and not C? Both atoms are part of the origin (CO bond), but only O is part of the destination (lone pair). The bond atom that is not part of the destination (C) is always the tail atom. Here is another example. The origin is a bond, but you should be able to pick out the tail atom (C) by finding the atom that is not part of the destination. The formal charges immediately follow from our rules: C is the tail (add +1), Br is the destination (add -1).
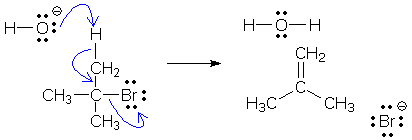
The next example shows a reaction with three arrows. Can you see that the arrows form a chain? The tail is O (add +1) and the head is Br (add -1). The other atoms are tail+head atoms and their charges do not change.
In some cases, a tail+head atom might start out with a positive or negative charge. This does not matter, however. As long as the atom is a tail+head atom, its formal charge will not change.
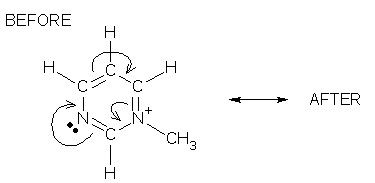
Review problems Take some time to practice what you have learned on this page. Work each problem in two steps. First, identify the tail and head atoms (if these atoms are present) in the BEFORE drawing and say what their formal charges will be in the AFTER drawing. Second, make a complete drawing of the AFTER formula and compare it with my answer. #1.
tail & head answers | AFTER answer
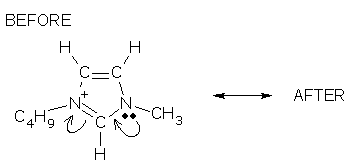
#2.
tail & head answers | AFTER answer
#3.
|