|
|

|
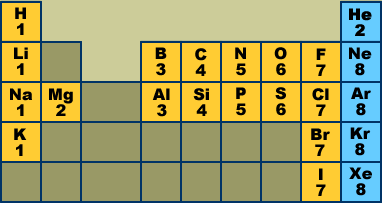
Lewis' Theory of Chemical Bonding Inert gas electron configurations (Lewis octets) The following Periodic Table shows some of the elements that appear most frequently in organic compounds. The numbers give the number of valence electrons held by each atom:
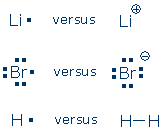
Do you see any patterns? Atoms in the same column have the same number of valence electrons. Atoms in the same row have varying numbers of valence electrons, but the number is always 8 or less. The inert gases (blue boxes) always hold 8 valence electrons (He is the exception). [1] Lewis postulated that inert gas atoms are chemically inert because they hold 8 valence electrons. The valence electron pattern in these atoms is called an inert gas electron configuration. Chemists also call this a Lewis octet. Lewis proposed that other atoms become more stable (less chemically reactive) by adopting an inert gas electron configuration. The atoms do this by ionizing (gaining or losing electrons) or by sharing an electron pair with another atom. Here are some familiar examples of "unstable" atoms (left) and chemically stable ions or molecules (right):
Review problems Questions #1-3 are based on the Periodic Table above. I expect you to know these electron configurations without having to consult the chart. Spend some time learning the chart at the top of this page, and then see how much of the following you can do. #1. Which inert gas does not have a Lewis octet? How many valence electrons does it have?
#2. Which atoms and/or ions can achieve inert gas electron configurations without achieving Lewis octets? Name them.
#3. For each atom listed below give a) the number of valence electrons it holds, b) the atomic ion that would have an inert gas electron configuration: O, Br, P, S, K, F, Mg.
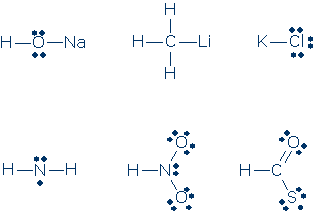
#4. Decide whether the atoms in these formulas have inert gas electron configurations (note: a given formula might contain a mix of atoms with and without proper configurations, therefore you need to check every atom). Every time you find an atom that lacks an inert gas electron configuration, say how many valence electrons are configured around this atom.
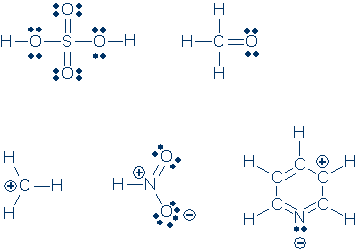
#5. Same as #4.
[1] Since most inert gases are chemically inert and their outer electrons are almost completely unaffected by neighboring atoms, one might argue that inert gases lack valence electrons. Thinking about things this way does not change things - atoms still try to adopt inert gas electron configurations - but it does make the phrase "Lewis octet" look odd ("Lewis null" might make more sense). [back] |